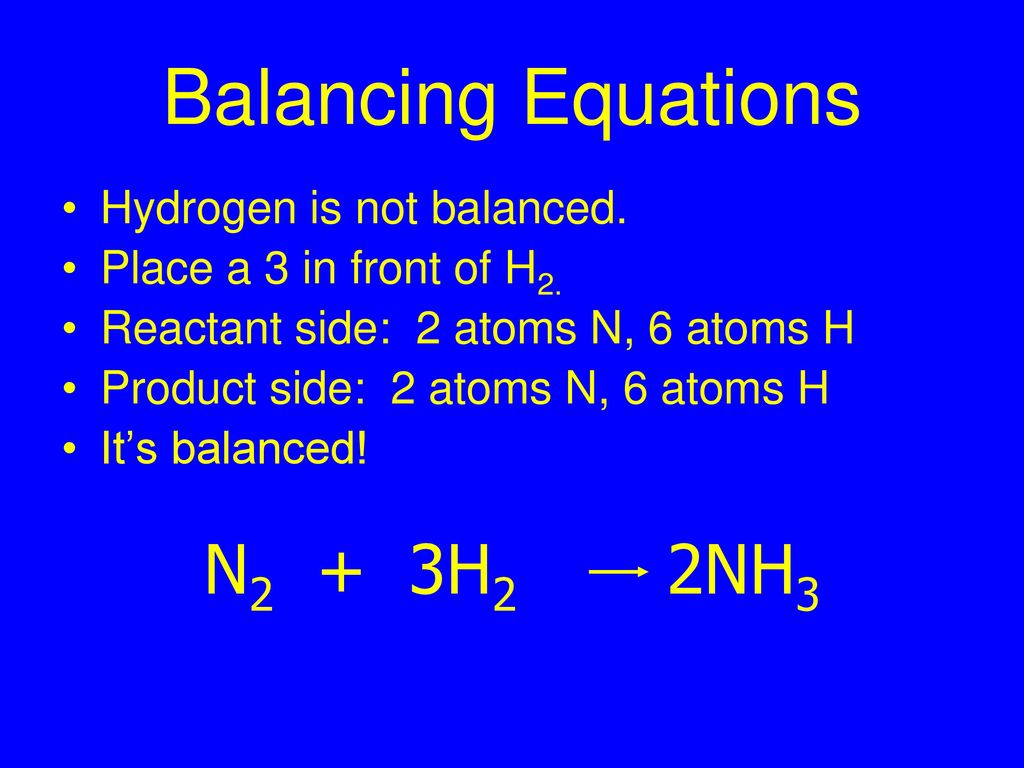
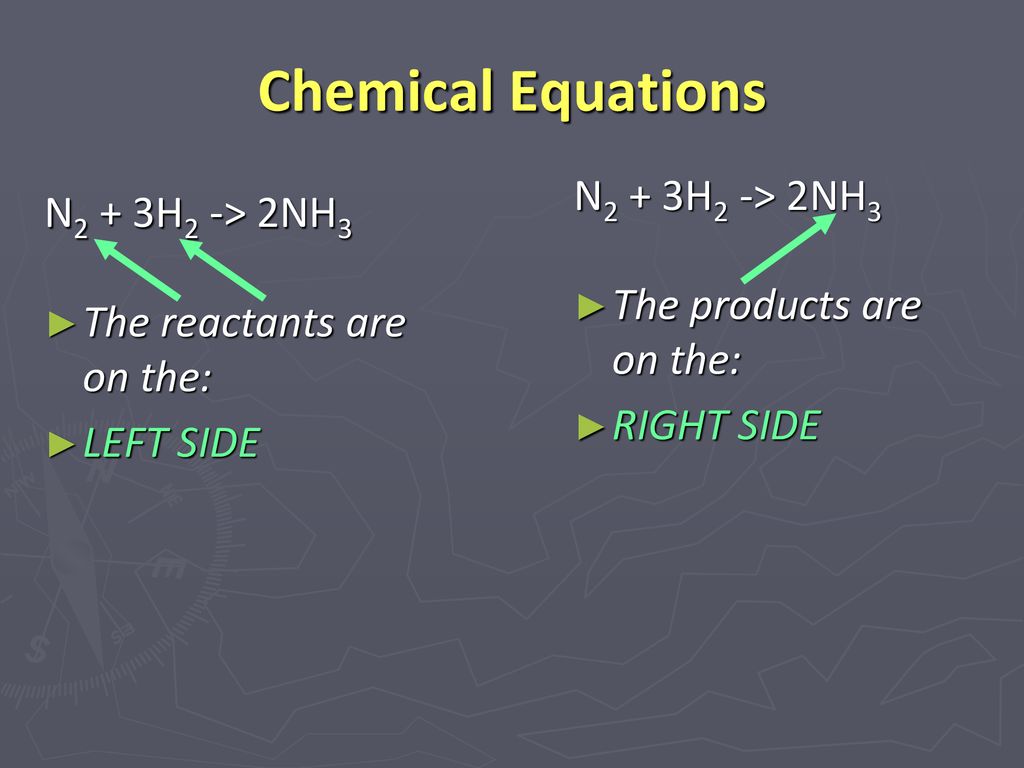
⚗️2NH. N2 + 3H2 Reactants Product On the balanced equation above, how many atoms of each element are - Brainly.com
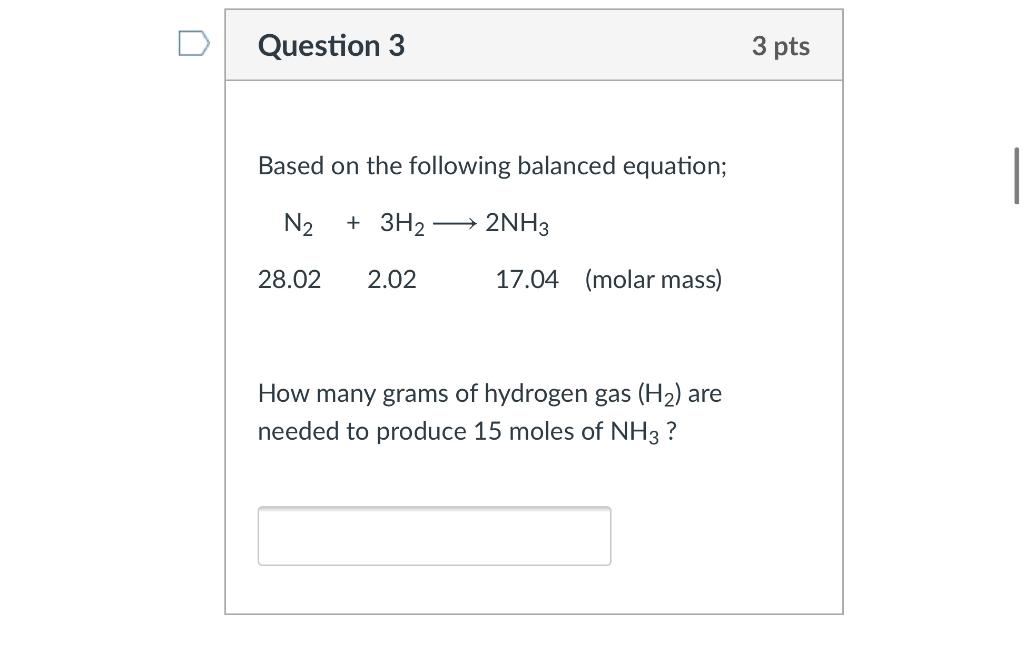
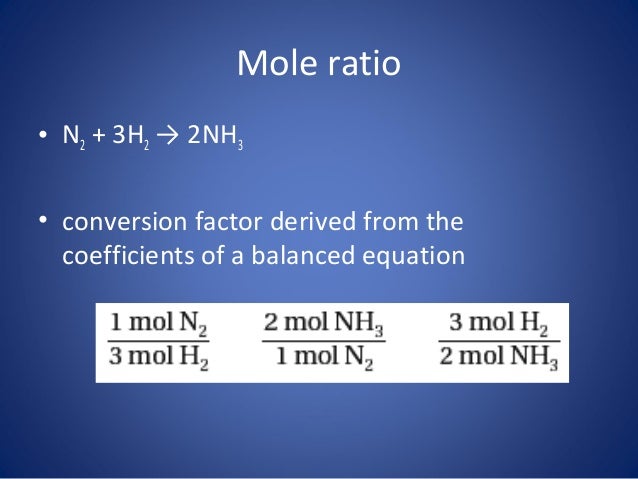
Consider the N2(g) + 3H2 (g) - 2NH3 (g), then how many moles of NH3 can be formed from 3.0 Mol N2 and 6.0 mol H2? - Quora
Solved] What is the balanced equation that represents the reaction between hydrogen and nitrogen to make ammonia (NH 3 )? | Course Hero
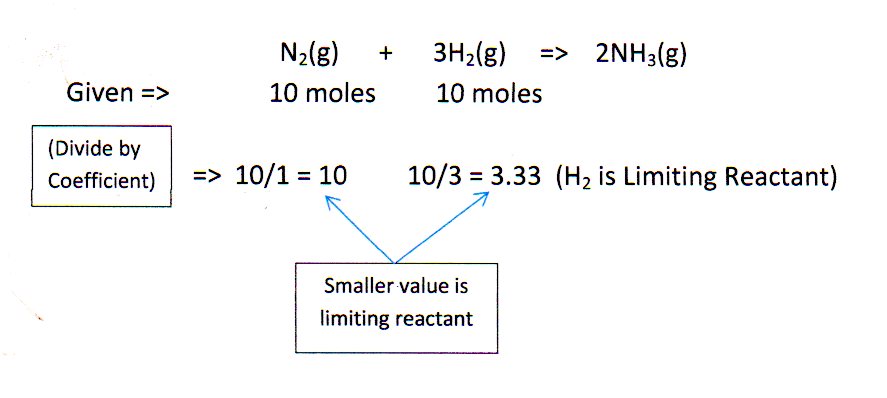
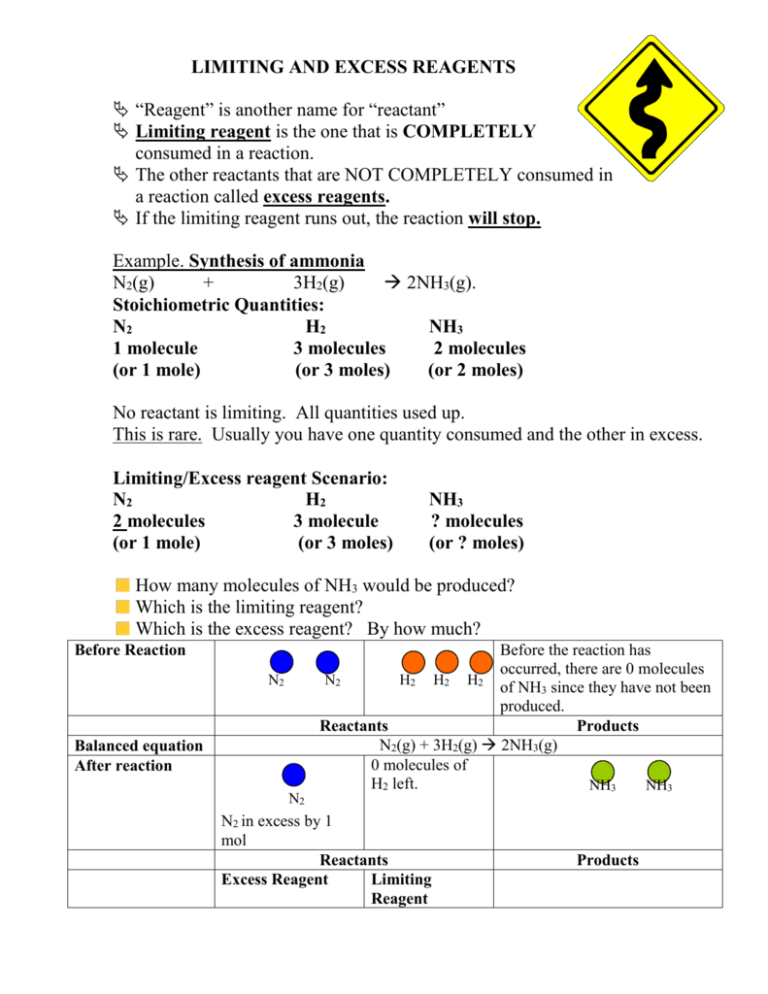
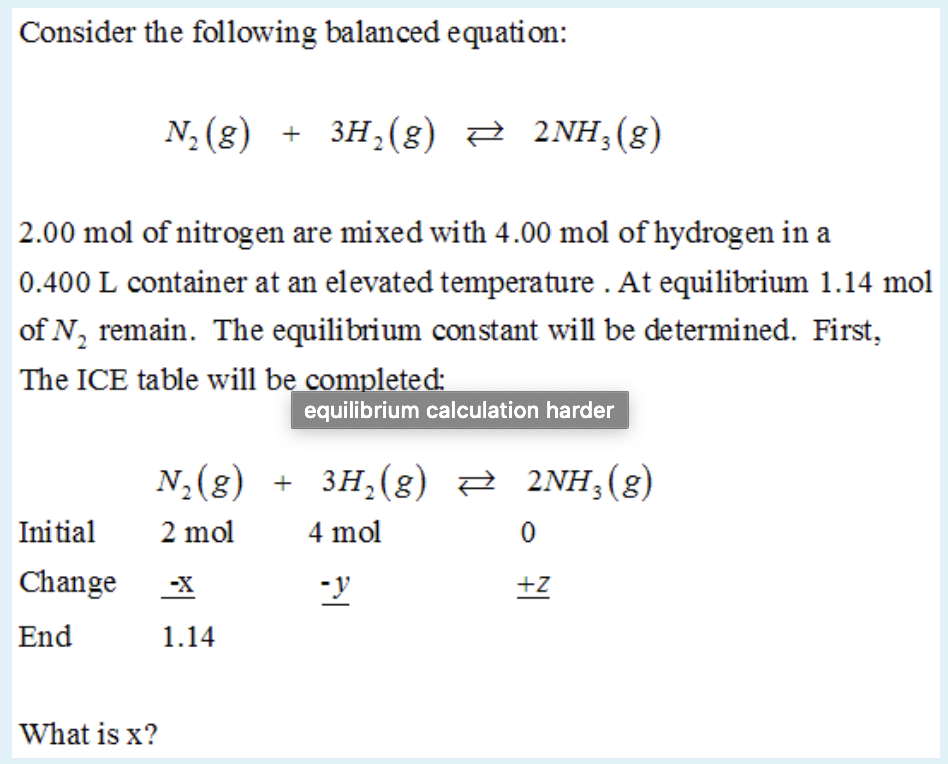
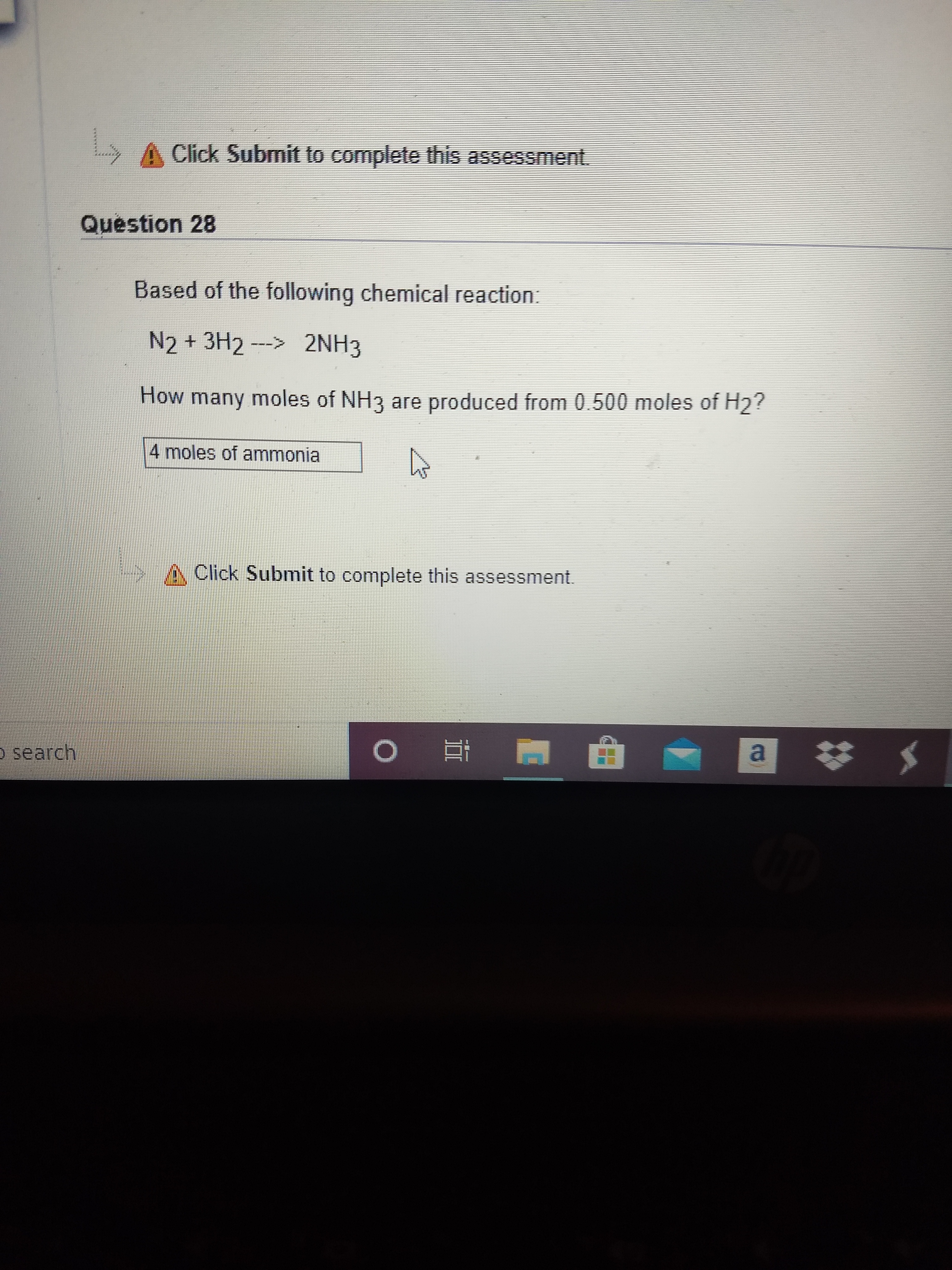
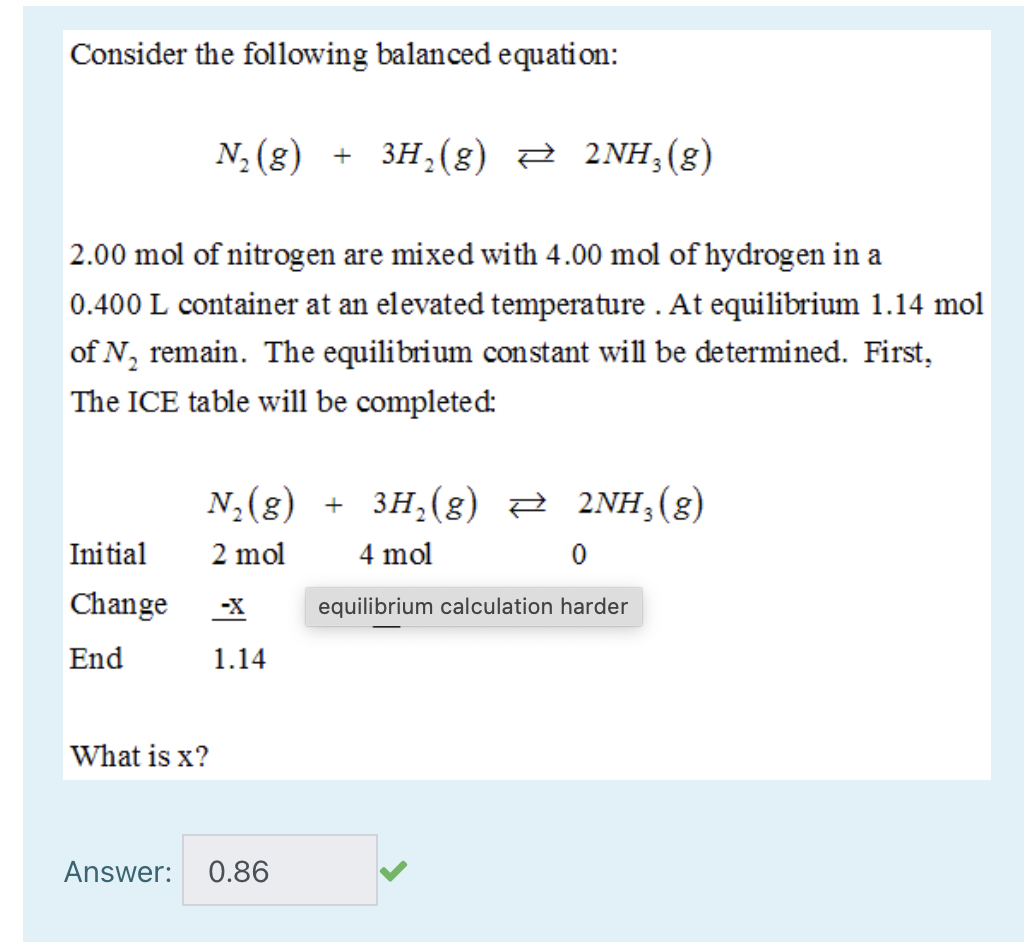
N_2 + 3H_2 -> 2NH_3. What is the limiting reactant if you start the reaction with 10.0 moles of each reactant? | Socratic
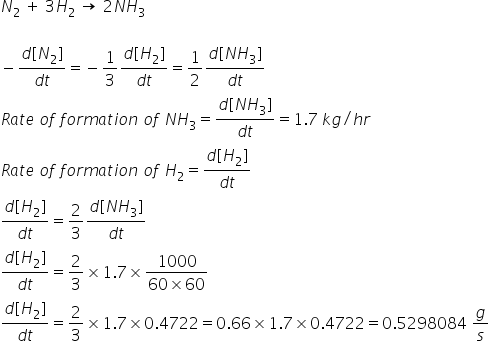
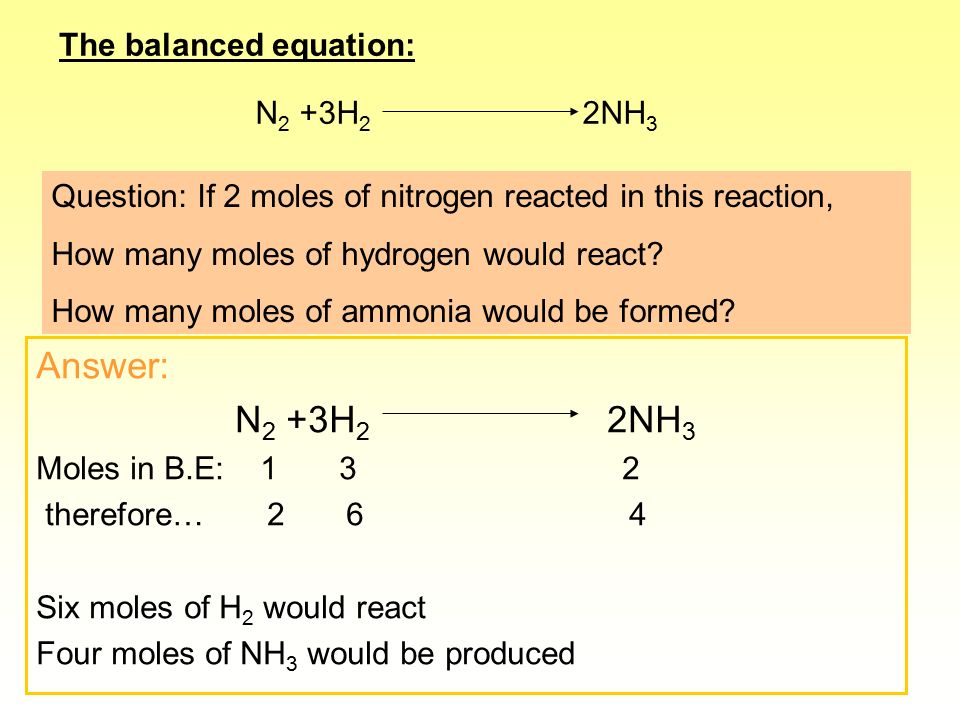
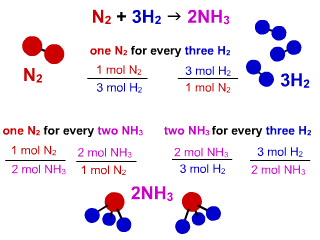
for a reaction n2 3h2 2nh3 rate of formation of nh3 is 17kg hour determine rate of concentration of h2 in g second - Chemistry - TopperLearning.com | hnb4tgg